
You may use the work for your own noncommercial and personal use any other use of the work is strictly prohibited. Except as permitted under the Copyright Act of 1976 and the right to store and retrieve one copy of the work, you may not decompile, disassemble, reverse engineer, reproduce, modify, create derivative works based upon, transmit, distribute, disseminate, sell, publish or sublicense the work or any part of it without McGraw-Hill’s prior consent. Use of this work is subject to these terms. (“McGraw-Hill”) and its licensors reserve all rights in and to the work. TERMS OF USE This is a copyrighted work and The McGraw-Hill Companies, Inc. For more information, please contact George Hoare, Special Sales, at or (212) 904-4069. McGraw-Hill eBooks are available at special quantity discounts to use as premiums and sales promotions, or for use in corporate training programs. Where such designations appear in this book, they have been printed with initial caps. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark. All trademarks are trademarks of their respective owners. 0-07-154220-5 The material in this eBook also appears in the print version of this title: 0-07-151136-9. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher. Manufactured in the United States of America. Sequential solution Simultaneous solution Multicomponent FlashĢ9 Binary Flash Overall mole balance mole balance on a mole balance on bģ0 Binary Flash Flash separation: Specify: Find: a = n-pentaneī = n-hexane Vfrac 0.471 V/F P 3.000 bar function -1.89E-08 Xa 0.394 Xb 0.606 Ya 0.619 Yb 0.Copyright © 2008, 1997, 1984, 1973, 1963, 1950, 1941, 1934 by The McGraw-Hill Companies, Inc. P = bar T = various oC Benzene - Toluene Y vs X diagram T vs X or Temp vs CompositionĢ6 Effect of Pressure: Seader & Henley (2006)Ģ8 Overview Brief thermodynamics review Binary Flash with energy balance Mixtures to model: MeOH – water EtOH – water propanol – water n-butanol – water n-butane – n-pentane benzene – toluene acetic acid – propionic acid CO2 – toluene (high P) Vapor phase: good liquid phase: good for HC or moderate – high pressures (compressible liquid phase) Typically used for liquid phase fugacity calculations Used for special cases (like gases dissolving into liquid phase) Equations of state models Activity coefficient modelsġ7 Overview Introduction UO course overview Equilibrium Stage separations

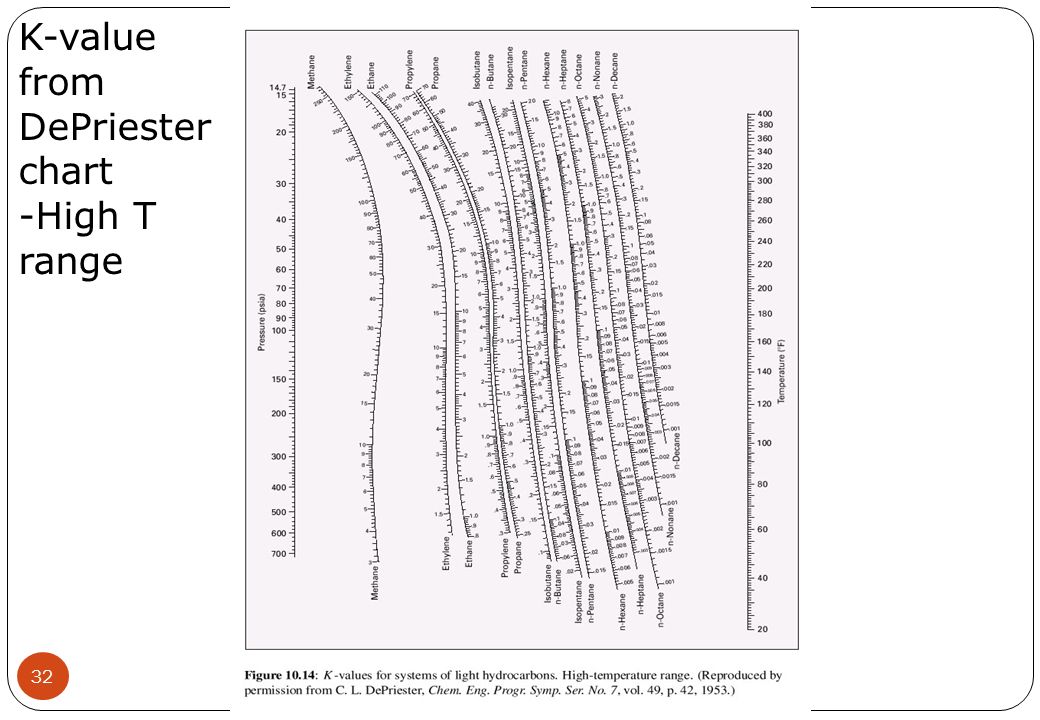
Historically, when estimates were done by hand: Sometimes the K values are nearly composition independent “hand” techniques of design/solution have used DePriester Charts (hydrocarbons):ħ DePriester Chart P = 2 bar T = 100 oC Isobutane others….

Historically, when estimates were done by hand: Seader & Henley (2006) Vapor phase: good liquid phase: good for HC or moderate – high pressures (compressible liquid phase) Typically used for liquid phase fugacity calculations Used for special cases (like gases dissolving into liquid phase) Typical simplifications: Ideal vapor phase Ideal liquid phase Raoult’s Law What are “Unit Operations” Brief thermodynamics review Binary flash with energy balance Multicomponent flash 2 Overview Introduction UO course overview Equilibrium Stage separations


 0 kommentar(er)
0 kommentar(er)
